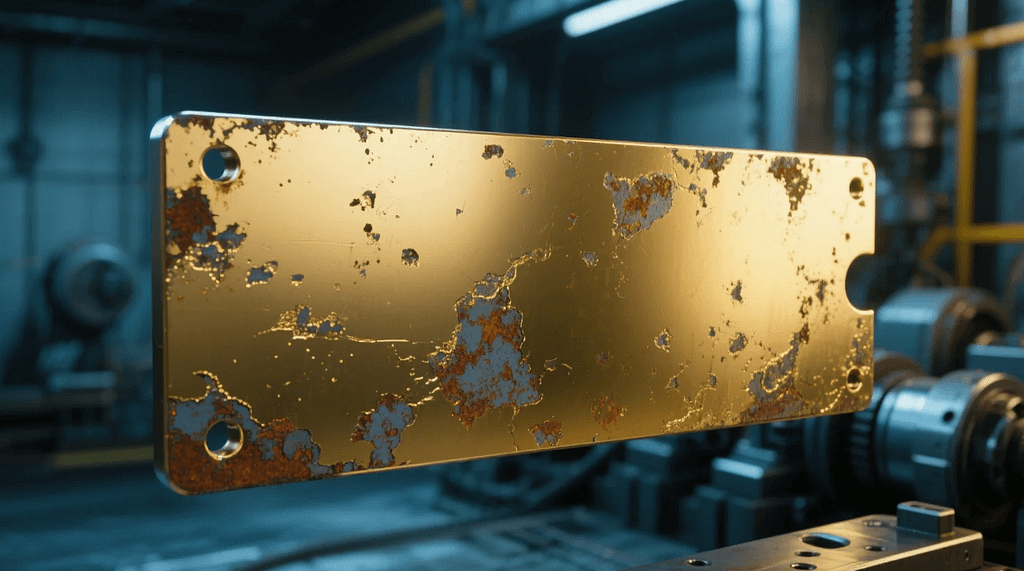
Introduction: Understanding the Truth About Does Gold Electroplate Tarnish
Among materials used in surface treatment and fine coating processes, gold electroplating holds a prominent place due to its visual appeal, conductivity, and resistance to corrosion. Yet a common question persists: does gold electroplate tarnish? This query is especially relevant in sectors like industrial modeling, where precision and material longevity are paramount. To answer this question with depth, it’s essential to understand the composition, properties, and conditions affecting gold electroplated surfaces.
What Is Gold Electroplating?
Gold electroplating is a surface finishing process in which a thin layer of gold is deposited onto a base metal (such as copper, nickel, or silver) using an electrolytic cell. The result is a decorative and functional surface that mimics solid gold while using far less precious material. The thickness of the gold layer can range from under 0.5 microns (flash plating) to several microns for higher durability.
Core Characteristics of Gold Electroplate
Conductivity
Despite being a thin coating, electroplated gold maintains excellent electrical properties, making it ideal for circuit connectors and sensor interfaces.
Non-Oxidizing Surface
Gold does not naturally oxidize or corrode, which is a key reason it’s widely used in high-performance components.
Aesthetic Appeal
Gold’s reflective and warm tone provides visual consistency and luxury appeal, even in technical mockups or composite applications.
Softness and Malleability
Gold is a soft metal; thin plating can wear down with friction unless sealed or reinforced with barrier layers.
So, Does Gold Electroplate Tarnish?
Technically, gold itself does not tarnish. However, does gold electroplate tarnish is not a simple yes or no question because the issue lies beneath the surface.
Factors That Influence Tarnishing:
- Base Metal Diffusion: If the underlying metal (like copper) diffuses through the gold layer, tarnishing or discoloration can occur over time.
- Gold Thickness: Thinner coatings are more vulnerable to environmental exposure and wear, leading to visible degradation.
- Surface Porosity: Micro-gaps in the coating can allow air or moisture to interact with the base metal.
- Environmental Conditions: Humidity, exposure to acids or salts, and air pollutants can exacerbate the degradation process.
Industrial Application Insight
In industrial modeling, where components may be stored for long periods or subjected to temperature fluctuations and chemical exposure, tarnishing becomes a practical concern. Although the gold layer itself is stable, the system around it may compromise the finish unless carefully engineered.
Advantages of Gold Electroplating in Industrial Modeling
Cost Efficiency
Compared to solid gold components, electroplating offers functional benefits without prohibitive costs.
Surface Precision
Allows tight control over thickness and area coverage, ideal for miniaturized models and contact points.
Corrosion Protection
Acts as a shield against atmospheric degradation of the base material, especially when appropriately layered.
Enhanced Bonding Capability
Facilitates soldering or adhesive bonding in multi-material prototypes.
How to Prevent Tarnish in Electroplated Gold
- Barrier Layers: Applying a nickel or palladium layer beneath the gold can slow down base metal migration.
- Sealing or Coating: Protective varnishes or anti-tarnish dips create a physical shield.
- Proper Storage: Using inert environments (e.g., vacuum packaging or desiccants) reduces exposure to tarnish-inducing elements.
- Controlled Handling: Wearing gloves and minimizing contact reduce contamination.
Comparisons: Gold Electroplate vs Other Platings
| Property | Gold Electroplate | Silver Plate | Nickel Plate |
|---|---|---|---|
| Tarnish Resistance | High (with layers) | Faible | Moderate |
| Electrical Conductivity | Excellent | Excellent | Poor |
| Appearance Longevity | Haut | Faible | Medium |
| Cost Efficiency | Moderate | Moderate | Haut |
Real-World Case Study: Gold Electroplating in Circuit Modeling
A prototyping lab involved in aerospace component design used gold electroplating for connector pins and signal paths in scale mockups. After six months of field simulation, untreated samples showed signs of tarnish due to copper migration. Conversely, parts with a nickel barrier layer and 2.5-micron gold plating remained pristine. This highlighted the need for multilayer planning in electroplated surfaces.
Innovations in Anti-Tarnish Gold Technology
- Nano-Sealed Layers: Engineered to reduce porosity and environmental exposure.
- Ion-Assisted Deposition: Improves adhesion and coating density.
- Smart Coating Diagnostics: Embedded sensors to monitor surface integrity in real time.
Final Thoughts: Why the Question Does Gold Electroplate Tarnish Deserves Context
In conclusion, the surface gold itself does not tarnish—but the underlying layers and application methods determine long-term stability. In industrial modeling, where every micron matters, understanding the interaction between gold, the base metal, and environmental exposure is critical. Proactive engineering, precise layering, and thoughtful storage practices can make gold electroplating a reliable and visually consistent solution for advanced applications.
FAQs – Gold Electroplating
Q1: How thick should gold electroplating be for industrial use?
A1: For durable performance, at least 1.5–2.5 microns is recommended, especially in active mechanical settings.
Q2: Can gold plating wear off?
A2: Yes, especially in high-friction environments or if the layer is too thin.
Q3: Is gold-plated better than solid gold for modeling?
A3: Yes, in terms of cost and weight, though it requires more careful handling.
Q4: Can tarnish be reversed once it appears?
A4: Minor tarnish can be polished off, but repeated cleaning may wear the gold layer.
Q5: Is gold electroplate recyclable?
A5: Yes, though the reclaim process is more complex due to thin layer recovery.