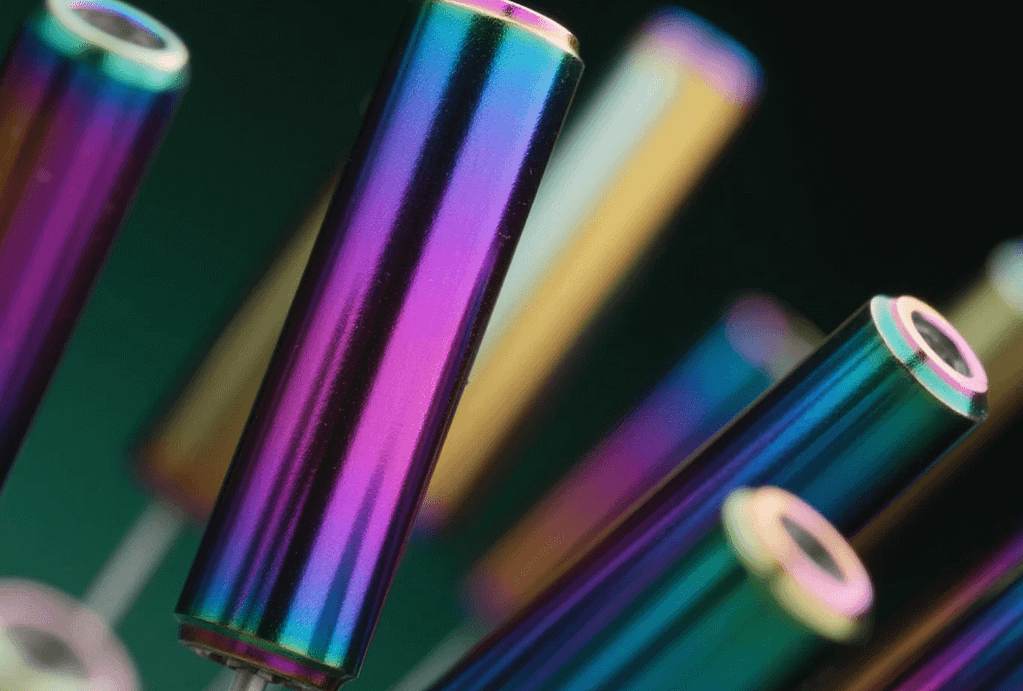
The Critical Choice: Surface Finishing in Precision Manufacturing
In the realm of high-stakes engineering and precision CNC machining, the difference between a successful component and a critical failure often lies in the quality of the surface finish. For manufacturers of complex, high-tolerance parts, particularly those fabricated from aluminum, the question of surface treatment is paramount. Therefore, anodized aluminum is not merely a coating; it is a fundamental transformation of the material’s surface, essential for meeting the stringent performance and aesthetic demands of industries like aerospace, medical devices, and high-end electronics.
This in-depth guide is designed to clarify the science, types, and crucial application considerations of anodized finishes, providing precision service users with the knowledge necessary to make informed decisions for their next project. By leveraging this sophisticated electrochemical process, precision-manufactured parts gain an unparalleled combination of durability, resistance, and visual quality. Ultimately, the integration of anodized finishing is a decisive step toward maximizing the lifespan and functional performance of your aluminum components.
What Exactly is the Anodizing Process?
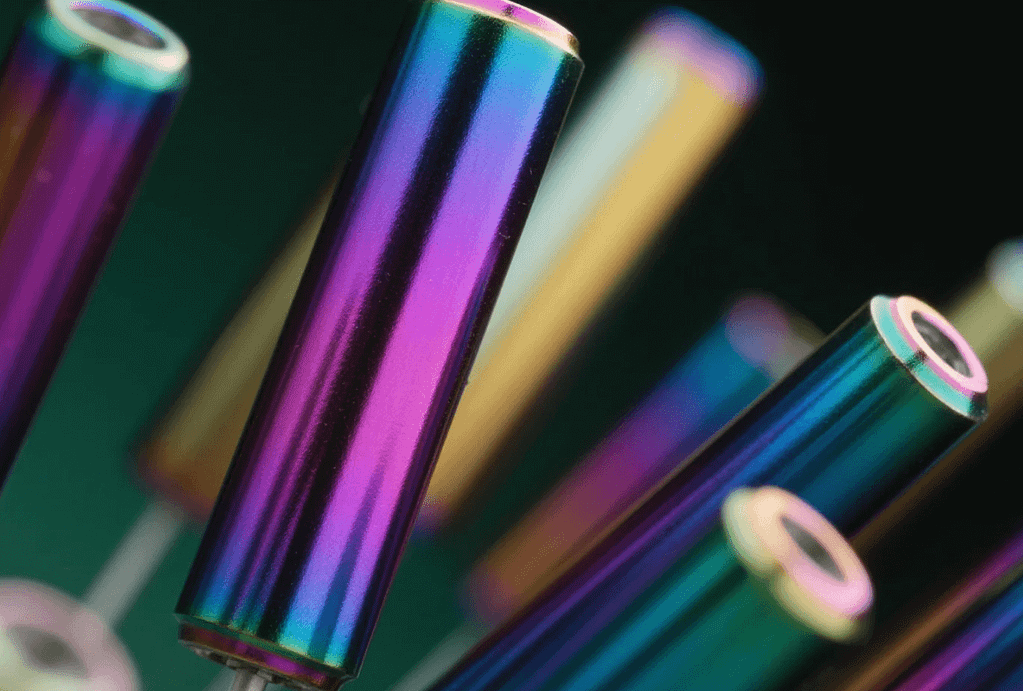
Anodized finishing is an electrochemical process, differentiating it significantly from mere plating or painting. Instead of applying an external layer, the process converts the metal’s native surface into a durable aluminum oxide layer. This oxide, fundamentally an integral part of the underlying aluminum, makes it exceptionally resistant to chipping, flaking, or peeling—a critical advantage in dynamic or high-wear applications.
During the process, the aluminum part is submerged into an acidic electrolyte bath, such as sulfuric acid. An electric current is then introduced, causing the aluminum to act as the anode (positive electrode), thereby triggering an oxidation reaction. Consequently, the metal surface transforms into a structured aluminum oxide layer. Furthermore, the thickness and density of this layer are meticulously controlled by adjusting the power supply and immersion time, thus meeting the exact specifications of the precision component. Following the creation of this porous oxide layer, a sealing process typically closes these pores, locking in color (if applicable) and maximizing corrosion resistance.
Enhancing Functional Integrity: Key Benefits of Anodized Finishes
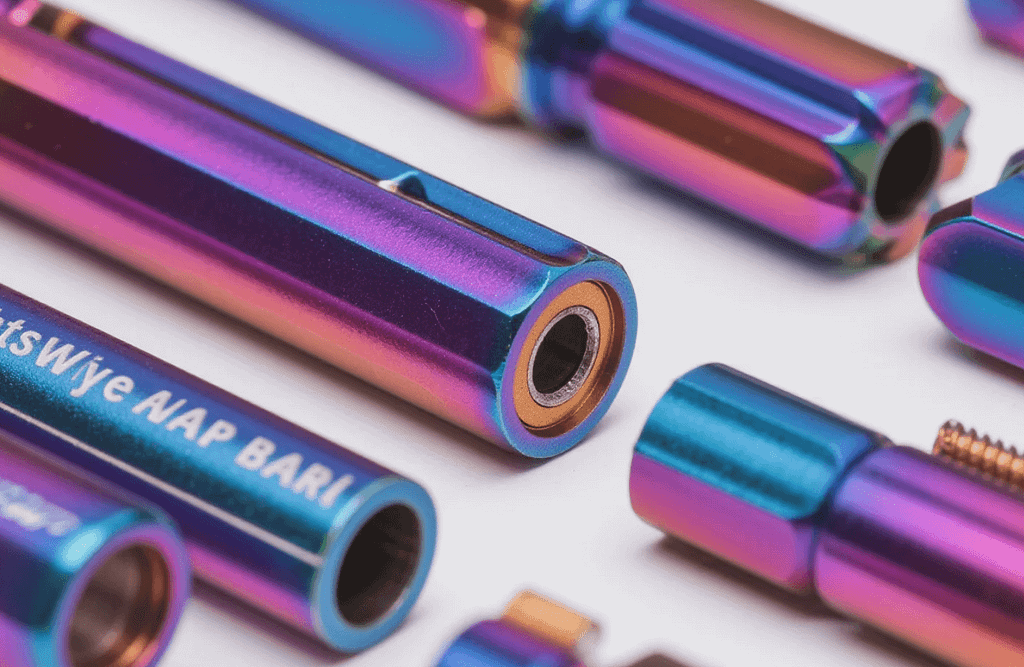
The shift from bare aluminum to an anodized state brings a suite of performance enhancements that are especially vital in the world of precision engineering.
Superior Corrosion Resistance
One of the most compelling reasons for choosing this finish is its dramatically enhanced resistance to corrosion. Aluminum naturally forms a thin oxide layer when exposed to air, but the anodized layer is exponentially thicker and more robust. This dense, non-reactive barrier successfully shields the underlying metal from moisture, saltwater, and harsh chemical environments, thereby ensuring the longevity and reliability of components used in marine, aerospace, and medical fields.
Unrivaled Wear and Abrasion Resistance
Specifically in the precision domain, parts often involve sliding, friction, or exposure to abrasive elements. While aluminum is inherently soft, the aluminum oxide created through anodized finishing is extremely hard—second only to diamond on the Mohs scale in some forms. This dramatically increases the surface’s resistance to wear, making it an essential treatment for gears, hydraulic components, pistons, and any part requiring exceptional surface durability.
Excellent Electrical Insulation
Unlike raw metal, the aluminum oxide layer is dielectric, meaning it acts as an electrical insulator. This property is crucial for electronic housings, internal chassis, and heat sinks where electrical isolation of a component is necessary to prevent short circuits or interference. The precisely controlled thickness ensures reliable and consistent insulating performance.
Optimal Adhesion for Subsequent Finishes
Due to its porous, uniform structure before sealing, an anodized layer provides an ideal surface for accepting dyes. This allows for clear color coding of precision parts, critical in complex assemblies for identification, safety, or purely aesthetic reasons, without sacrificing any of the functional benefits. The resulting colors are saturated, durable, and fade-resistant.
The Spectrum of Anodizing: Choosing the Right Type
Not all anodized finishes are created equal. Precision manufacturing utilizes several distinct types, each offering a specific trade-off between film thickness, hardness, and aesthetic appeal.
Type II (Sulfuric Acid Anodizing – Clear and Color)
Type II is perhaps the most common and versatile. It generates a relatively thin (typically 0.0002″–0.001″ / 5–25 $\mu$m) and aesthetically pleasing finish. This is the choice for applications where appearance is important, such as consumer electronics, decorative panels, or architectural elements, but where moderate abrasion resistance is still needed. Importantly, Type II coatings accept a wide variety of colors.
Type III (Hardcoat Anodizing – Hard Anodized)
Known as Hardcoat, Type III is the powerhouse of the anodized finishes. Using a lower temperature and higher current density, it produces a significantly thicker and harder coating (often 0.002″ / 50 $\mu$m or more). This process penetrates the substrate as much as it builds up on the surface. Consequently, Hardcoat is specified for components subjected to extreme wear, high-friction environments, and demanding military or aerospace applications where dimensional stability must be factored in. It typically results in a dark gray or bronze color, often un-dyed, prioritizing rugged performance over decoration.
Chromic Acid Anodizing (Type I)
Type I produces the thinnest oxide layer (typically less than 0.0001″ / 2.5 $\mu$m). Its primary advantage is minimal dimensional change on complex parts and excellent corrosion resistance without significantly impacting the part’s fatigue strength. It is frequently employed for non-dyed, highly critical aerospace components.
Design and Manufacturing Considerations for Precision Parts
For precision manufacturers like Captec Precision, integrating anodized finishing requires rigorous attention to design and process details. A truly professional service partner will guide users through these crucial factors:
- Dimensional Impact: It is essential to recognize that the anodized layer adds material to the surface. For example, a Type III Hardcoat layer of 0.002″ (2 mils) will effectively thicken the part’s dimension by approximately 0.001″ per surface, as the process consumes about half of the layer thickness from the base metal. Tolerances for precision fits must be calculated with this build-up in mind.
- Alloy Selection: The final anodized finish quality is heavily dependent on the aluminum alloy. Alloys like 6061 and 7075 are generally excellent for anodizing, producing clear, consistent results. Conversely, high-silicon casting alloys can result in a muddier, less predictable finish. Selecting the correct alloy is the first step toward a successful outcome.
- Contact Points (Racking): Since anodized finishing requires an electrical current, the part must be physically contacted or ‘racked.’ A professional finisher will select small, non-critical surfaces (e.g., inside a blind hole or on a surface that mates with another part) for these contact points. The area under the rack mark will not be anodized. This crucial consideration must be discussed during the design phase to ensure no critical tolerance surfaces are compromised.
The Value-Driven Choice in Precision Machining
Ultimately, choosing an anodized finish is a clear demonstration of a commitment to quality, durability, and a profound understanding of material science. For users procuring high-tolerance, custom CNC machined components—whether for satellites, diagnostic equipment, or robotics—the investment in a professional anodized process pays dividends. It safeguards the meticulous work of the machining process, protects against environmental and mechanical degradation, and delivers a superior product that reflects correct engineering values. Therefore, when performance is non-negotiable, the selection of a Type II or Type III anodized finish, applied by a partner with expertise in precision requirements, transforms a quality part into an exceptional, long-lasting solution.
Frequently Asked Questions (FAQ)
Q1: How does anodizing affect the dimensions of my precision part?
A: Anodizing adds material to the surface. In Type III Hardcoat, about half of the total film thickness is growth and half is penetration. You must account for the growth factor in your initial machining tolerances. For example, a 0.002″ thick coat will add about 0.001″ to each surface dimension.
Q2: Can I get an anodized finish on steel or stainless steel parts?
A: No, the anodized process, as described, is specific to materials like aluminum (and, less commonly, titanium and magnesium) that form an oxide layer. Steel and stainless steel require different surface treatments, such as passivation, electroplating, or powder coating, for corrosion and wear resistance.
Q3: What is the primary difference between Type II and Type III (Hardcoat)?
A: The main difference is thickness and hardness. Type III is significantly thicker, harder, and more wear-resistant, often specified for functional, high-stress components. Type II is thinner, offers moderate protection, and is widely used when a cosmetic color is a key requirement.
Q4: Will the anodized color fade over time?
A: High-quality, properly sealed anodized finishes are very resistant to fading, particularly those using inorganic dyes or integral color processes. However, prolonged, direct exposure to harsh UV light can cause some organic dyes to fade over many years. Black and natural finishes are the most fade-resistant.